 Illustration of isochoric chamber that supercools heart tissue to subfreezing temperatures without ice formation. (Image by Anthony Consiglio/UC Berkeley)
Illustration of isochoric chamber that supercools heart tissue to subfreezing temperatures without ice formation. (Image by Anthony Consiglio/UC Berkeley)Cold-hearted science: Supercooling technique advances preservation of human tissue
Researchers at UC Berkeley successfully revived human heart tissue after it had been preserved in a subfreezing, supercooled state for 1 to 3 days.
By preserving the cardiac tissue at a constant volume in a rigid isochoric chamber, researchers were able to prevent the formation of ice crystals that could have damaged the micro heart muscle cells. The researchers examined the structural integrity of the heart cells and tested whether the tissue retained normal functions, such as autonomous beating and responsiveness to drugs and external electrical stimuli.
The results, described in a paper published Sept. 22 in the journal Communications Biology, are a key proof-of-concept of supercooled tissue cryopreservation.
The method they used, called isochoric supercooling, was pioneered in the lab of Boris Rubinsky, UC Berkeley Professor of the Graduate School at the Department of Mechanical Engineering, professor emeritus of bioengineering and a senior author of the study.
“To our knowledge, this is the first-ever report of the supercooling and revival of an autonomously beating, engineered human cardiac muscle,” said study co-lead author Matt Powell-Palm, a postdoctoral scholar in Rubinsky’s lab.
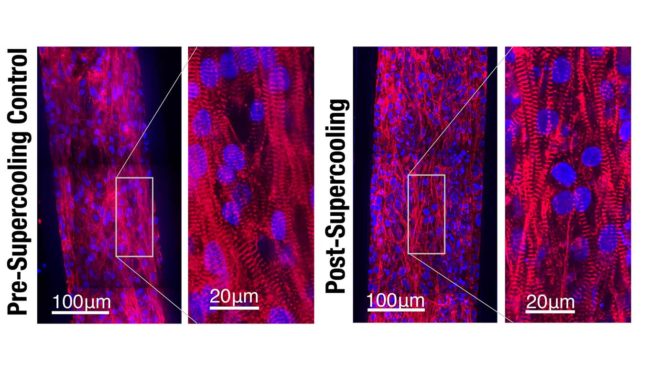
Confocal microscope images show that the integrity and alignment of the sarcomeres, or muscle filaments, in engineered human heart tissue were preserved after supercooled preservation. (Image by Matt Powell-Palm, UC Berkeley)
Rubinsky noted that these results have near-term implications for the preservation and transport of organ-on-a-chip platforms, expanding access beyond the select few labs that can manufacture them for research and industry. “Such platforms are valuable tools for the design of new drugs for diseases of the heart and other organs,” he said.
The results also suggest that isochoric supercooling may be a viable technique in the future for preserving donor tissue and organs, a daunting challenge facing the transplantation community and the medical research field, the study authors said. The researchers cited estimates that seven out of 10 thoracic donor organs are discarded each year because of the inability to preserve them long enough to reach patients in need. The viability of a donor heart, for instance, is measured in hours, greatly limiting the number of potential recipients who could benefit from a lifesaving transplant.
“Currently, patients in Florida cannot receive a heart or lung from California because the organ would not survive the cross-country trip,” said Powell-Palm.
Addressing those challenges is a key goal of the Advanced Technologies for the Preservation of Biological Systems (ATP-Bio) Engineering Research Center, funded last year by the National Science Foundation. Kevin E. Healy, professor of bioengineering and of materials science and engineering, is the UC Berkeley lead of ATP-Bio and another senior author of this study.
Giving no space for ice
The constant-volume state that is characteristic of isochoric freezing requires the sample to be kept in a liquid in a sealed, air-free rigid container. Such conditions yield no space for cell-damaging ice to form, even at temperatures that fall below freezing.
This differs from conventional isobaric freezing, in which material is in contact with the atmosphere at a constant pressure. Samples are frozen solid under atmospheric pressure, a process that also takes more energy.
Rubinsky noted that in previous studies, they have been able to cool samples down to minus 22 degrees Celsius while keeping 40% of the material unfrozen. “This is fundamental thermodynamics. When the material to be frozen is confined in a rigid box, then only part of the volume freezes,” he said.
Rubinsky, Powell-Palm and scientists at the U.S. Department of Agriculture recently showed that isochoric freezing could be applied to the food industry, leading to better quality preservation of food and reduced energy consumption compared with conventional freezing methods.
The researchers hold a UC patent on isochoric supercooling, and Powell-Palm is the CEO of BioChoric Inc., a startup working on clinical translation of this technique.
Heartwarming results
For this study, the researchers used cardiac tissue grown from adult stem cells, a heart-on-a-chip system that was developed in Healy’s lab in 2015. The cardiac tissue beats at a rhythm comparable to a human heart, and the system’s microfluidic channels replicate the way the cells are exposed to nutrients and drugs.
“It’s not enough to say that these biological samples survived supercooling,” said Healy. “We wanted to demonstrate that physiological and metabolic function remained largely intact.”
The researchers submerged the heart-on-a-chip in a rigid chamber filled with a common organ preservation solution that had been chilled to minus 3 degrees Celsius. They removed the heart cells from the solution after durations of 24, 48 or 72 hours, and returned them to a warm 37 degrees Celsius.
Examination of the heart tissue confirmed that isochoric supercooling had not altered the structural integrity of the heart tissue, nor did it significantly affect the beat rate or beat waveform. The study noted that there was a slight upward trend in the duration of the heartbeat with longer supercooling periods, but the physiological impact of that change was not clear.
They found that spontaneous beating resumed for 65% to 80% of the human heart muscle chips that had been supercooled for up to three days. Moreover, they found no significant difference resulting from the duration of supercooled preservation.
“We consider these percentages relatively high for preservation recovery results, especially considering the intrinsic variability in these heart-on-a-chip platforms and the fact that no cryoprotectants were used,” said Powell-Palm.
The researchers also found that after emerging from cryopreservation, the heart tissue remained responsive to isoproterenol, a medication that results in an increased heart rate.
The researchers emphasized that further work is needed to scale up these results to full organs.
“The technology used to cool the tissue is sound and robust, but we now need to develop techniques to warm things up consistently,” said Healy. “That was easier with the mini heart muscles we used for this study. Working on whole organs will require more work.”
Other study authors from Healy’s Biomaterials and Tissue Engineering Laboratory are co-first author Verena Charwat, a postdoctoral researcher; Berenice Charrez, who recently earned her Ph.D. in bioengineering; and researcher Brian Siemons.