Machines that heal

Imagine a tiny, wirelessly-powered device that can be safely implanted in the brain to restore useful functions for people disabled by a spinal cord injury, stroke or cerebral palsy. A micromachine that could also be used to prevent epileptic seizures — or even treat symptoms of Parkinson’s or Alzheimer’s diseases.
Recent breakthroughs in brain-machine interfaces have brought medical advances such as these to the verge of reality. And Rikky Muller, assistant professor of electrical engineering and computer sciences (EECS) and co-director of the Berkeley Wireless Research Center, is at the forefront of this miniaturized revolution in healthcare. She is using her expertise in integrated circuits to build tiny, implantable devices that are intelligent, safe and so minimally invasive that they can last over the course of a patient’s lifetime.
“I’m absolutely passionate about finding treatments and maybe someday cures for neurological diseases,” says Muller. “Almost everyone knows someone impacted by neurological diseases like epilepsy, Parkinson’s or Alzheimer’s. That’s why this is such an important area to invest time and resources into.”
Neurological diseases are notoriously hard to treat, and patients are typically prescribed pharmaceuticals for these conditions. But if they don’t respond to drug treatment, there aren’t many other options.
“This idea totally changed my perspective on healthcare — to have a device implanted that can alleviate symptoms and give patients back their quality of life, especially for extremely difficult disorders,” says Muller. “I want to build devices that can help these patients.”
Building brain-machine interfaces
Muller was working in the semiconductor industry, designing integrated circuits for consumer devices, when she first learned about the potential health benefits of brain-machine interfaces. Knowing that her expertise could be applied to building these devices, she began to voraciously read scientific papers and attend conference presentations on the subject. Eventually, with bachelor’s and master’s degrees in electrical engineering from MIT, she enrolled in Berkeley’s EECS doctoral program, with a specific focus on integrated circuits and neuroengineering.
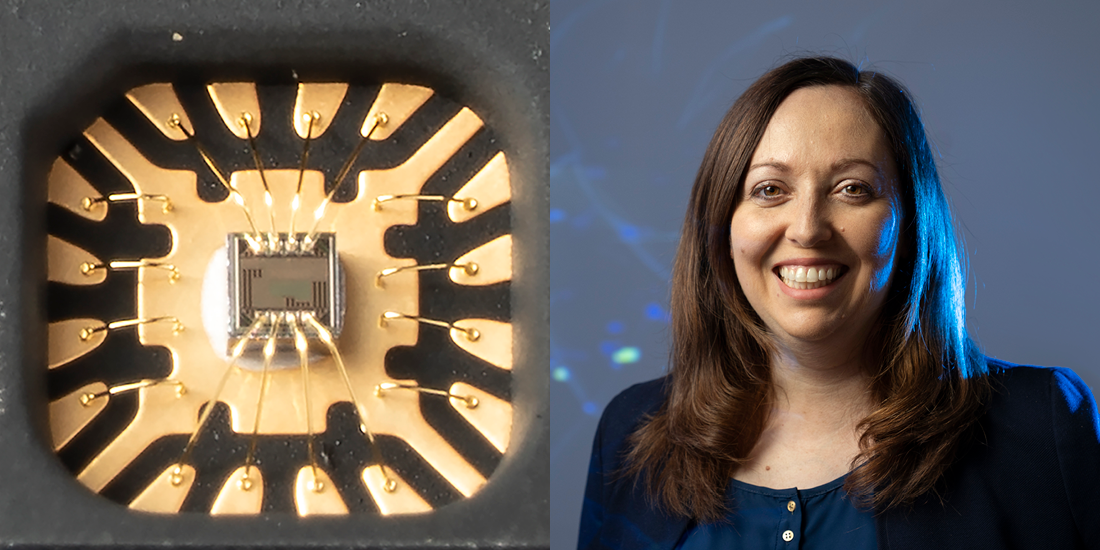
For her thesis, Muller worked closely with collaborators around campus, including EECS professors Jan Rabaey and Michel Maharbiz (Ph.D.’03 EECS), to build a micro-electrocorticography (ECoG) device. This small implant — as thin as plastic wrap and as flexible as a soft contact lens — can record neural activity and then transmit the data wirelessly for analysis, using ultra-low power circuits and a microfabricated electrode array.
“Rikky…helped us overcome a number of obstacles, so our devices could be significantly smaller and use less power, but still have wireless capabilities, data acquisition, application and noise filtering.”
– Jan Rabaey, EECS professor
According to Rabaey, this device was among the first in the field with wireless capabilities, which is important because it allows the surgical site to be closed after the device is implanted in the body, effectively minimizing the risk of infection. The high-density ECoG electrode sensors can also map regions of the brain’s cortex with high resolution. The sensors eventually became the first product of Cortera Neurotechnologies, a company co-founded by Muller, Maharbiz, Rabaey and Peter Ledochowitsch (Ph.D.’13 BioE) and incubated in the CITRIS Foundry. Muller became Cortera’s first chief executive officer in 2013, and later, its chief technology officer.
“Before this, people had been doing circuits for brain integration, but their devices were substantially larger and used a lot more power without having the wireless interface,” says Rabaey. “Rikky is a really good circuits designer, and she helped us overcome a number of obstacles, so our devices could be significantly smaller and use less power, but still have wireless capabilities, data acquisition, application and noise filtering.”
Rabaey admits that many researchers didn’t initially realize the importance of making something really small. The density that Muller achieved allowed them to fit many additional electrodes in a device, allowing researchers to collect much more information. Her contributions also made these implants extremely power-efficient, running on microwatts per channel.
Stimulating neural dust
Muller also played an important role in advancing the technology behind neural dust. Invented by a collaboration of researchers — including EECS professors Rabaey, Maharbiz, Jose Carmena and Elad Alon — neural dust was the first wireless, dust-sized sensor powered by ultrasound. Not only does ultrasound charge the device, it’s also used to transmit data.
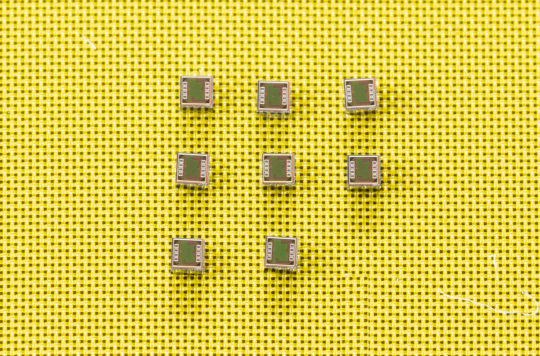
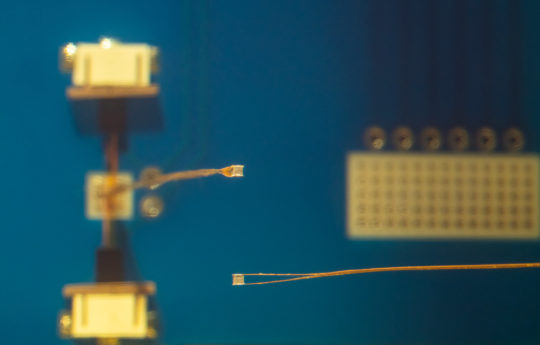
Muller helped to develop the neural dust platform by building an advanced wireless neural recorder that can record nerve or neural activity with a high degree of accuracy. Her team achieved this by designing a custom integrated circuit to transfer ultrasound charge to the nerve in a well-controlled and safe way. The device is called StimDust, short for stimulating neural dust.
“When most people think of wireless devices, they think of WiFi or Bluetooth as standard protocols. But we have to create our own protocols that make things extremely energy efficient.”
– Rikky Muller
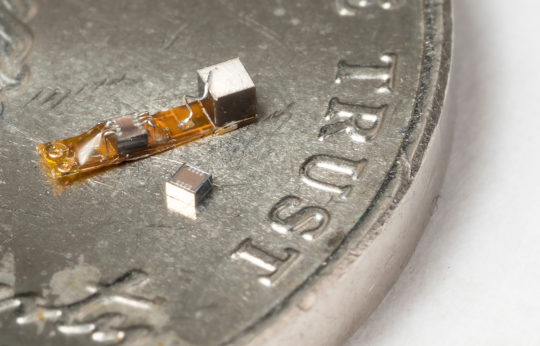
In addition to building the platform, Muller also designed a custom wireless protocol that gives researchers a large range of programmability. The entire device is just 1.7. cubic millimeters in volume — the size of a grain of sand. That’s an order of magnitude smaller than comparable active devices with similar capabilities.
“When most people think of wireless devices, they think of WiFi or Bluetooth as standard protocols. But we have to create our own protocols that make things extremely energy efficient,” says Muller. “This is not a generic radio that can transmit any type of data; it’s communicating something very specific, and since there’s no place for data storage, we have to design custom chips to communicate safely and efficiently.”
Muller hopes that one day StimDust will treat diseases like heart irregularities, chronic pain, asthma or epilepsy. She and her colleagues are also optimistic that neural dust can someday be implanted noninvasively, like through a syringe, and transmit data to a wearable device to monitor internal nerves, muscles and organs in real time.
When her team implanted a StimDust device on a cuff around the sciatic nerve in a rodent, they were able to control hind-leg motion, record the stimulation activity and measure how much force was exerted on the hind leg muscle as it was stimulated. By gradually increasing the stimulation, they were able to map the response of the hind leg muscle, so they knew exactly how much stimulation was needed for a desired muscle movement.
She also helped build upon the original dust concept to create a 0.8 cubic millimeter device that can safely, precisely and wirelessly record from a peripheral nerve or neuron. Researchers can communicate with multiple implanted devices at once, which would allow them to record networks of neural activity in 3D. This has never before been demonstrated in such a minimally invasive manner.
Pacemakers for the brain

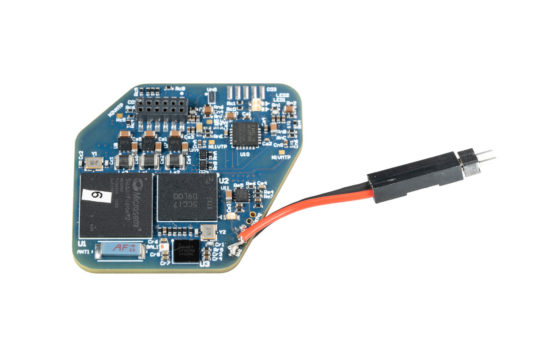
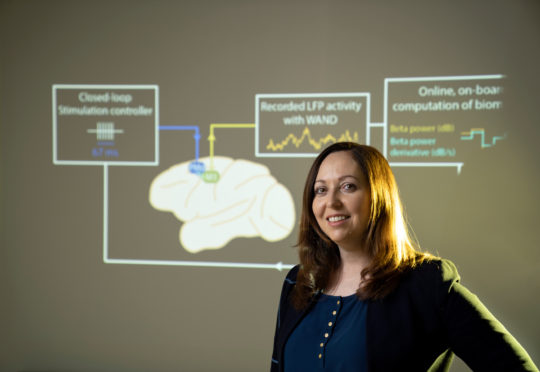
The latest innovation that Muller has worked on is WAND, short for wireless artifact-free neuromodulation device. Comparable to a pacemaker for the brain, this device can monitor the brain’s electrical activity and deliver electrical stimulation if it detects something wrong.
WAND is based on technology developed in a 2014 project that included researchers from Berkeley and Cortera. Muller began building on this work when she returned to Berkeley in 2016 as an assistant professor.
Because WAND is both wireless and autonomous, it could be trained to recognize the signs of a tremor or seizure, and adjust the stimulation parameters on its own to prevent the unwanted movements. Since the device is also a closed-loop system — meaning it can stimulate and record at the same time — it can adjust its stimulation parameters in real time based on a patient’s neural state. As a proof of concept, researchers were able to recognize and delay movements in rhesus macaques.
“We wanted to enable the device to figure out what is the best way to stimulate for a given patient to result in the best outcomes. And you can do that by listening and recording the neural signatures,” says Muller.
In the future, Muller and her team hope to incorporate learning into their closed-loop platform to build intelligent devices that can figure out how to best treat patients and remove the doctor from having to constantly intervene in this process. “This area of research is all about rethinking the traditional. The devices that I create take a long time to develop because we’re building these things from scratch,” says Muller. “For me, the most exciting part of the process is seeing everything come together and work as it was designed to.”