
Precious cargo
The use of nanoparticles to deliver chemotherapy drugs to cancerous tumors started in the 1980s. Since then, researchers have experimented with different styles of nanocarriers to hit upon the right combination of size and materials to release drugs to the heart of tumors without punishing side effects.
Too large, and the nanocarriers cannot penetrate a tumor cell for a direct hit. Too small, and they get lost in the bloodstream before finding the target. Besides, introducing a material into the body that cannot get out again may cause side effects that negate the benefits of treatment.
Researcher Ting Xu has conceived of a solution that greatly advances the science and offers promising clinical implications for cancer patients and for sufferers of other diseases and conditions.
Using a soap-like material whittled down to a neat 15 nm size, Xu has developed a nanocarrier that slips into a tumor cell to release treatment where it can have its most powerful effect. The nanocarrier then dissolves into small bits that pass quickly and safely through the body without toxic accumulation.
“I look at this as a beautiful marriage of basic science and real-world application,” says Xu, who holds faculty appointments in materials science and engineering and chemistry.
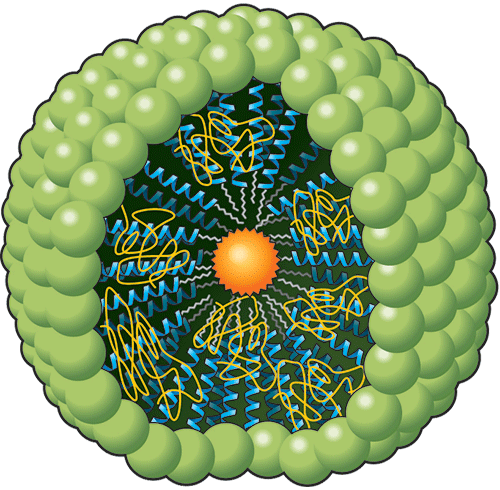
This distinct drug carrier combines different nanotechnologies to achieve its small size and ability to exit the body safely. The design crosses one of the leading ingredients in laxatives, polyethylene glycol (PEG), with soap’s chemical structure. The result is a peptide-polymer particle that is able to circulate longer in the bloodstream and leaks less cargo than other types of nanocarriers.
Indicator: Driven by polarity, the heads of these molecules bind together, while the helix tails (in blue) tuck inside, forming a sphere called a micelle. Attached to the tails are the hair-like PEG polymers (in yellow).
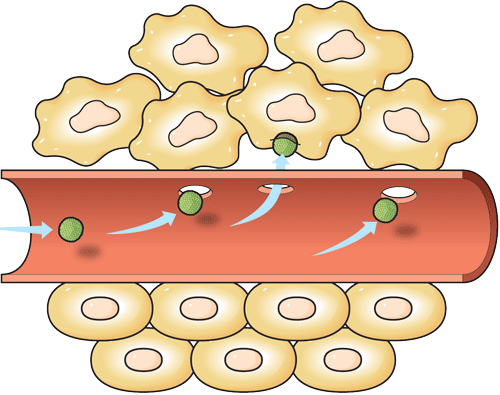
Researchers found that the optimal size for a drug nanocarrier to travel well through blood vessels and infiltrate a tumor is between 10 and 30 nm. The carriers Xu has tested are 15 nm in diameter. Currently, FDA-approved nanocarriers are 100-130 nm in size.
Indicator: Healthy tissue is made up of uniform, tightly packed vascular spaces. Cancerous tumors grow chaotically, making them more permeable. If sized correctly, the drug carrier will enter the tumor passively.
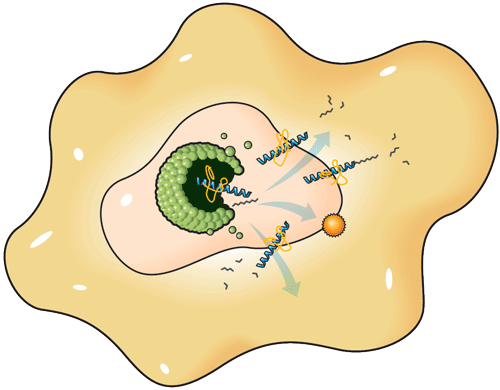
Delivering and dispersing the drug cargo to all parts of a tumor is critical to preventing regeneration. Once inside the tumor, the nanocarriers are broken down by the cell’s own debris-clearing function and the drug is released from the carrier.
Indicator: Inside the tumor, the cell’s waste-eating enzymes break the bonds holding the micelle together, releasing the drug within.
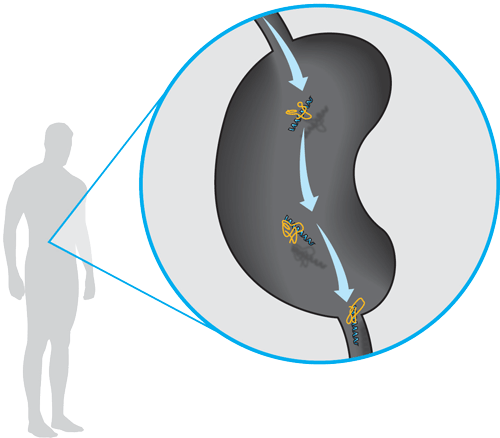
After delivering the drug, the nanocarrier breaks down into its original components, which can pass through the kidneys. “After they do chemo, a lot of people have liver failure because the clearance pathway is not right,” Xu says.
Indicator: The parts of the carrier become small enough to leave through the urinary tract without causing infection or damage.

