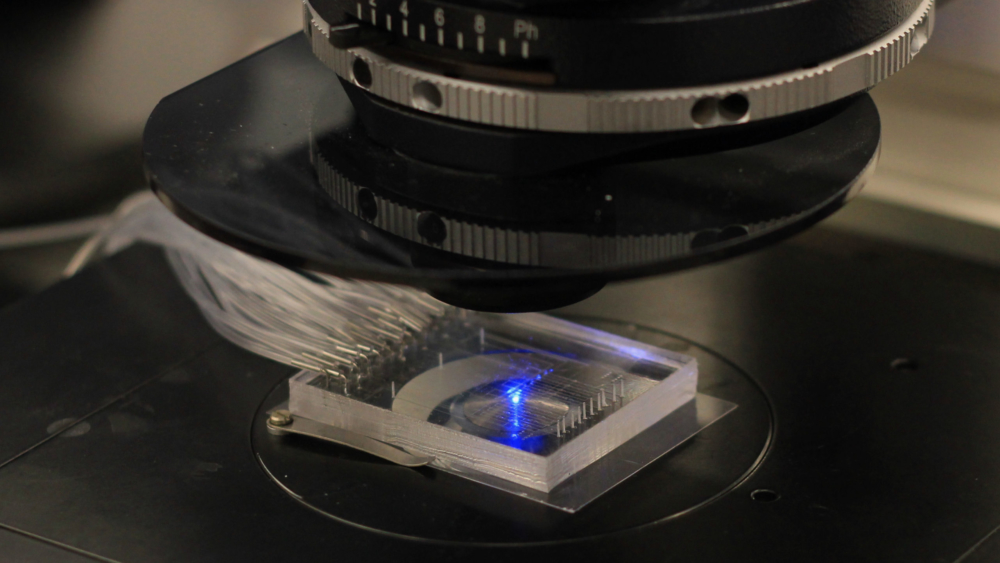
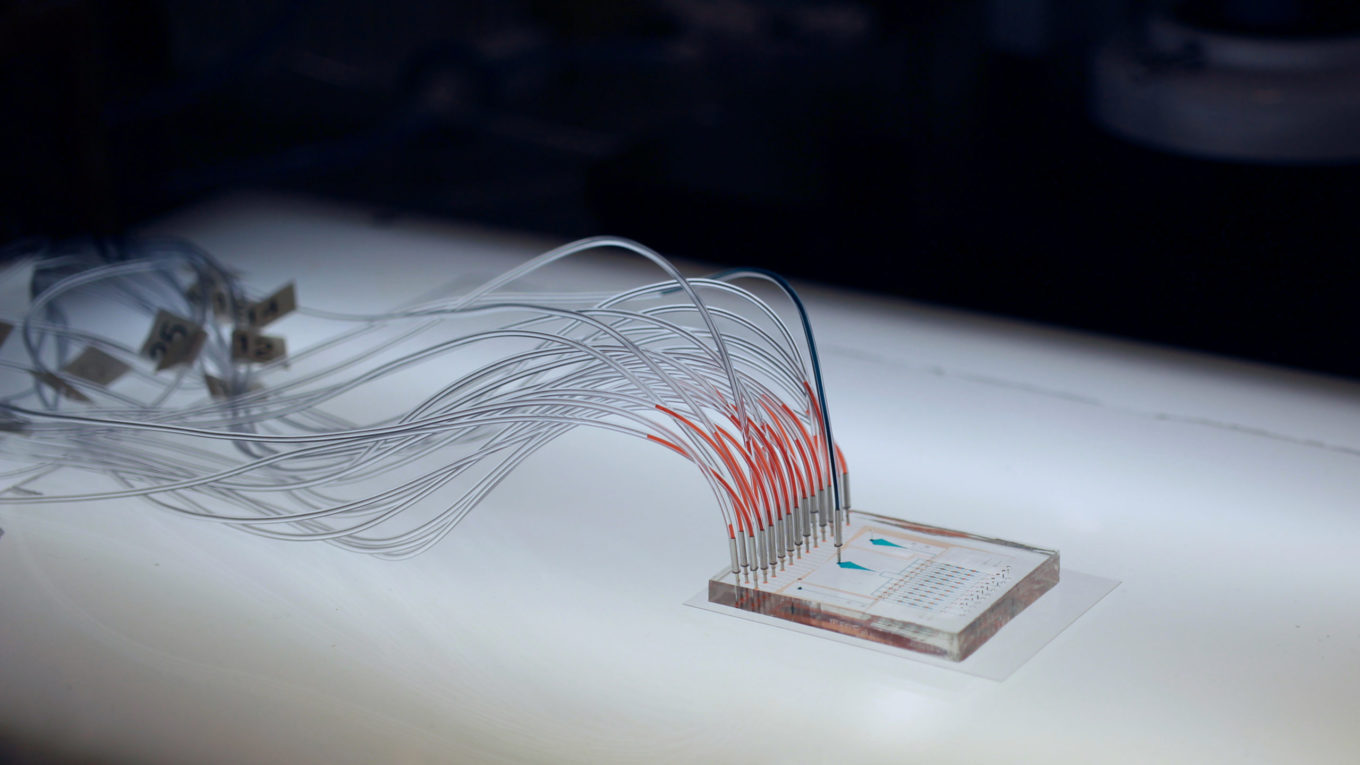
 A microfluidic "chip" developed by the Streets lab for single cell analysis
A microfluidic "chip" developed by the Streets lab for single cell analysisUsing microfluidics to peer deeper into the structure of our genome
Imagine untangling a ball of yarn hundreds of feet long and trying to re-wind it in exactly the same way — that’s essentially what a cell does with its DNA every time it divides. Now, new work in assistant professor Aaron Streets’ bioengineering lab attempts to make sense of this process. In a paper published in the journal Cell Systems, the UC Berkeley researchers describe a novel technique, based on a microfluidic platform, for unraveling and imaging lengthy strands of DNA.
Many important discoveries have relied on the ability to take high-resolution microscope images of biological processes occurring in single cells. While microscopy still represents the gold standard for validating where molecules are in a cell, more recent high-throughput DNA sequencing technologies have allowed researchers to sequence the DNA and RNA from single cells, providing rich information about the information that is encoded in our genome.
Pairing these imaging and sequencing measurements expands the toolkit available to study biological questions in single cells and, specifically, to learn how genomic information is related to what a cell looks like. This is especially useful for studying processes that differ among individual cells. But until now, it’s been very difficult to take a picture of a single cell with a microscope and then pick that cell from the microscope slide and sequence its DNA.
The Streets’ lab’s new technology, called 𝛍DamID (or MicroDamID), sets up an innovative approach for the joint imaging and sequencing of protein-DNA interactions in single cells. Through a microfluidic platform, 𝛍DamID can trap a single cell — using light-microscopy to “see” how the DNA is organized in the nucleus — and then apply DNA sequencing to read exactly which sequences are where inside of it.
Measuring DNA’s spatial and linear organization
Nearly every cell in the human body contains identical copies of six feet of DNA coiled up and tightly packaged into a nucleus with a diameter less than one-tenth of the width of a human hair, all while keeping the DNA highly organized and tangle-free. Exactly how this packaging is arranged helps to determine which genes are turned on and off in different cell types, affecting how stem cells differentiate and how certain cancers develop. At the single-cell level, researchers are just now beginning to understand that the way the DNA is organized makes that cell different from its neighbor.
“It’s wound into this incredibly sophisticated ball of yarn and packed into the nucleus of every cell in your body,” Streets said. “If you were to read your genome, that sequence encodes information to make you a human, but it also makes you different from me. The way that ball of yarn is wrapped, it also stores information that tells each given cell what to be. If the genome is packed this way, it’s a skin cell; if it’s packed that way, it’s a muscle cell. This packaging is related to what’s called ‘epigenetics.’” There are roughly 30,000 genes in the human genome, and where they’re located in the nucleus is an important clue into what makes each cell function in a particular way.
“MicroDamID is a technique that takes a picture to ‘see’ where proteins are binding to DNA inside the cell, then sequences the DNA at those same binding sites from the same single cell to reveal where they are in the genome,” said bioengineering Ph.D. student and lead author Nicolas Altemose. “It’s hard enough to do one or the other, but this tool allows us to measure and understand both the spatial and the linear organization of the DNA. It improves our ability to create these protein-DNA binding maps to study how DNA is regulated in the nucleus.”
Possible applications of the technology are abundant. The process of meiotic recombination, for example, is essential for fertility and is responsible for shuffling around DNA before it’s packaged into sperm or eggs. Imaging and sequencing protein-DNA interactions in those cells would greatly improve our ability to study the exact molecular mechanisms of meiotic recombination. Another process that occurs uniquely in single cells is errors in chromosomal segregation. Imaging single cells at high resolution before sequencing would make it possible to answer key questions about that process.
From an empty lab to ‘aha’ moments
The 𝛍DamID project began in 2016 with an empty lab and some startup funds, Streets said. Altemose, his first Ph.D. student, and Jonathan White, his first research technician, built the lab and developed its microfluidics infrastructure with the help of Paul Lum and the Biomolecular Nanotechnology Center in the California Institute for Quantitative Biosciences (QB3) at UC Berkeley. “There were many ‘aha’ moments as well as some ‘oh no!’ moments,” Streets said. “The big one for us was when Nick was first able to amplify DNA from a single cell in the microfluidic device. As soon as he got that to work, we knew MicroDamID was technically possible.”
Now, four years later, the researchers’ initial idea is a working technology. “It’s an exciting demonstration of how engineering at the intersection of microfabrication, microscopy and DNA sequencing can produce new tools that make multiple precision measurements on single cells,” Streets said, “allowing us to peer deeper into the molecular machinery of the fundamental units of life.”
Other study co-authors include Annie Maslan, Carolina Rios-Martinez, Andre Lai and Jonathan A. White. Funding from the National Institutes of Health and the Chan Zuckerberg Biohub helped support this research.