 Berkeley engineers have developed a thermal regulator that could be used to easily extend the temperature range of battery-powered devices, including smartphones, laptops and electric vehicles. (iStock photo)
Berkeley engineers have developed a thermal regulator that could be used to easily extend the temperature range of battery-powered devices, including smartphones, laptops and electric vehicles. (iStock photo)Berkeley ingenuity keeps lithium-ion batteries at temps even Goldilocks would love
New research by Berkeley engineers may soon make it more practical to use battery-powered vehicles and devices in extreme temperatures, such as in icy-cold winters in Minnesota or stifling-hot summers in Death Valley. Those conditions represent temperature ranges that fall outside the narrow window — typically 20 to 40 degrees Celsius — needed for a lithium-ion battery’s optimum and safe performance.
“The optimum temperature range for lithium-ion batteries may not be a serious issue in the mild climate of the San Francisco Bay Area, but in the middle of winter in New York or Lake Tahoe, it’s not unusual for smartphones to automatically switch off because it’s too cold,” said Chris Dames, a UC Berkeley professor of mechanical engineering and head of the research team that developed a new thermal regulator — described in a study in the journal Nature Energy — that could resolve this problem.
The researchers explained that a battery’s usable energy drops dramatically in cold temperatures. At minus 20 degrees Celsius, a typical commercial lithium-ion battery cell can deliver only 20 percent of its room-temperature capacity.
High temperatures can also create problems for batteries, which generate their own waste heat when in use. Battery lifetimes typically halve for every 13 degrees Celsius of excess temperature.
“What’s worse is that overheating can lead to ‘thermal runaway,’ a failure mode that can lead to the battery fires in electric cars as well as certain mobile phones and electronics that we’ve heard about in the news,” said study lead author Menglong Hao, a postdoctoral researcher in Dames’ lab.
Managing conflicting temperature needs has been a challenge for thermal packaging. Current methods that keep batteries at their preferred temperatures consume energy and are too expensive or bulky to include in many portable applications. In contrast, the new thermal regulator developed by UC Berkeley engineers keeps batteries at stable temperatures through a passive system that does not consume extra energy.
“Active heating and cooling cost energy, energy that you don’t want to spend on keeping the battery comfortable when you could’ve used it to drive another 50 miles,” said Dames.
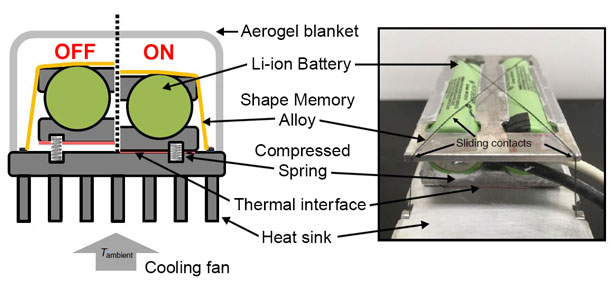 Shown is a schematic and photo of a thermal regulator designed by Berkeley engineers to passively keep lithium-ion batteries within an optimum temperature range. At higher temperatures, wires made of a shape memory alloy hold batteries down close to a heat sink so that excess heat is dissipated. At lower temperatures, the wires loosen so that the resulting air gap helps the batteries retain heat. (Image courtesy of Menglong Hao, UC Berkeley)The passive system uses a shape memory alloy, a class of material that is characteristically soft and pliable at low temperatures, but hardens back to its original shape at higher temperatures. Such materials are commercially available and routinely used in medical implants.
Shown is a schematic and photo of a thermal regulator designed by Berkeley engineers to passively keep lithium-ion batteries within an optimum temperature range. At higher temperatures, wires made of a shape memory alloy hold batteries down close to a heat sink so that excess heat is dissipated. At lower temperatures, the wires loosen so that the resulting air gap helps the batteries retain heat. (Image courtesy of Menglong Hao, UC Berkeley)The passive system uses a shape memory alloy, a class of material that is characteristically soft and pliable at low temperatures, but hardens back to its original shape at higher temperatures. Such materials are commercially available and routinely used in medical implants.
The exact temperature of the transition from soft to hard depends on the mix of the metals. In this case, the researchers chose nickel and titanium alloy wires that transitioned at 35 degrees Celsius. Below that threshold, the wires softened, but above 35 degrees Celsius, the wires stiffened and contracted.
The researchers attached the wires to a lithium-ion battery pack such that the “on” position was at the higher temperatures, with the stiffened wires pulling the batteries tightly into contact with a heat sink designed to cool the batteries down. At temperatures below 35 degrees Celsius, the softened wires were in the “off” position, allowing the battery pack to lift away from the heat sink with the help of compressed springs. The resulting air gap provided insulation that helped keep the batteries warm by slowing the dissipation of their own waste heat.
The researchers tested the thermal regulator in both vacuum and real-world conditions to confirm that their system could move easily between hot and cold states.
At a cold ambient temperature of minus 20 degrees Celsius, they demonstrated that their thermal regulator could increase the battery temperature to 20 degrees Celsius just by retaining the battery’s self-generated heat. At the same time, at a hot ambient temperature of 45 degrees Celsius, the thermal regulator kept the batteries from overheating by limiting the temperature rise to about 6 degrees through constant heat dissipation.
“All of this was accomplished passively, without any sensors, logic or electrical power consumption,” said Hao. “Another benefit is that the shape memory alloy wires are cheap, running only 1 percent of the total cost of the battery, so this is a cost-effective system.”
In a commentary published in Nature Energy, Carnegie Mellon University professors Jonathan Malen and Venkat Viswanathan said the thermal switch developed at UC Berkeley could improve upon technologies other than batteries that are sensitive to fluctuating temperatures. Those include fuel cells, sensors and lasers.
The UC Berkeley researchers said that their thermal regulator system could have major implications for wider adoption of renewable technologies. Lithium-ion batteries power a host of consumer products ranging from electric cars and drones to laptops and smartphones. The batteries also play a key role in stabilizing the electricity grid against fluctuating solar and wind generation by powering renewable energy storage units.
The study authors noted that out of the 51 U.S. metropolitan areas with populations over 1 million, 20 typically experience temperatures that fall below zero degrees Fahrenheit, and 11 areas have summer temperatures that routinely rise above 100 degrees Fahrenheit.
“By inventing a new type of thermal regulator, we came up with a single design that can work for both Lake Tahoe in January and Death Valley in August,” said Dames.
Other UC Berkeley study co-authors include Scott Moura, professor of civil and environmental engineering; Saehong Park, a graduate student researcher in Moura’s lab; and Jian Li, a visiting scholar from the School of Energy and Environment at Southeast University in China.
Funding from the Toyota Research Institute North America helped support this research.
