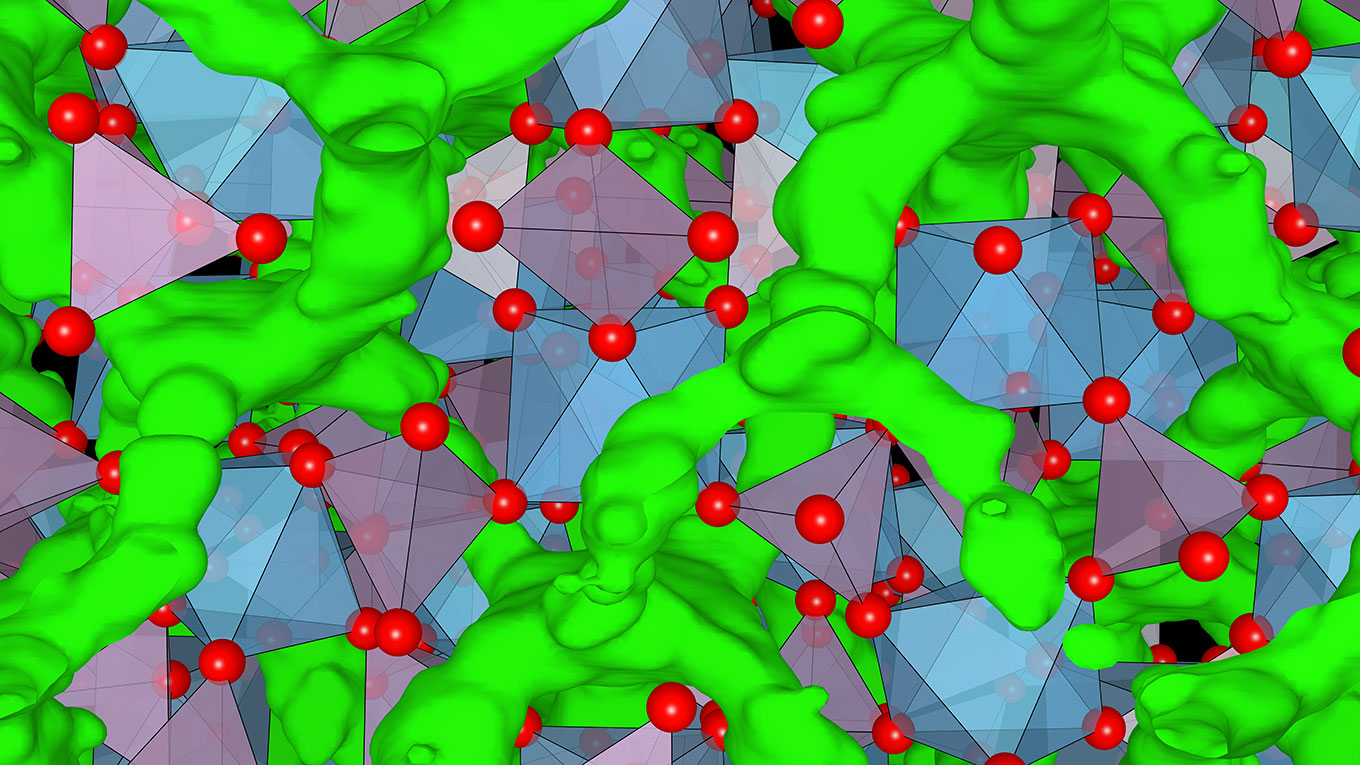
 Illustration of the crystal structure of a lithium superionic conductor with corner-sharing frameworks. Green areas show lithium ions diffusing through the framework of non-lithium cations (polyhedrons) to achieve high ionic conductivity. (Image courtesy of Ceder lab.)
Illustration of the crystal structure of a lithium superionic conductor with corner-sharing frameworks. Green areas show lithium ions diffusing through the framework of non-lithium cations (polyhedrons) to achieve high ionic conductivity. (Image courtesy of Ceder lab.)Researchers discover pathway to safer lithium-ion batteries
Rechargeable lithium-ion batteries power everything from portable devices to electric vehicles, but the liquid electrolytes inside them are combustible, posing a potential safety risk if these batteries overheat or become damaged. Berkeley engineers have now identified a new class of materials that may be the key to developing a safer lithium-ion battery.
Battery researchers have been developing solid-state batteries to address this concern and provide a more stable alternative, but lower ionic conductivity in solid-state electrolytes can impede performance, posing a barrier to adoption.
In a study published in Nature Materials, a research team led by Gerbrand Ceder, professor of materials science and engineering, discovered a new approach to developing solid electrolytes that may solve the conductivity issue.
“Lithium ions in a solid electrolyte must be able to move as fast as they do in liquid electrolytes, but this property is rare in solid materials, especially in oxides,” said Ceder. “Now we have a much better understanding of how we can design a fast lithium ionic conductor.”
Using a theory- and data-driven approach, the researchers discovered that they could achieve high lithium ionic conductivity in a specific group of oxide-based materials. “We identified a special structural feature in these materials called corner-sharing frameworks, which promotes lithium ionic conductivity,” said KyuJung Jun, a Ph.D. candidate in Ceder’s lab.
They then searched the open-access Materials Project database at Berkeley Lab and identified other materials with this corner-sharing frameworks feature, which maximizes conductivity by providing structural pathways through which lithium ions can quickly diffuse.
“We discovered 10 new oxide structural frameworks that have superionic lithium conductivity,” said Jun. “This vastly expands the collection of materials that can be used to create solid-state batteries that are potentially safer and still high performing.”
Other study authors are Yingzhi Sun, a Ph.D. candidate in Ceder’s lab, and Yan Wang, a scientist from Samsung.
