Berkeley bioengineers discover how tumor cells can mimic Velcro
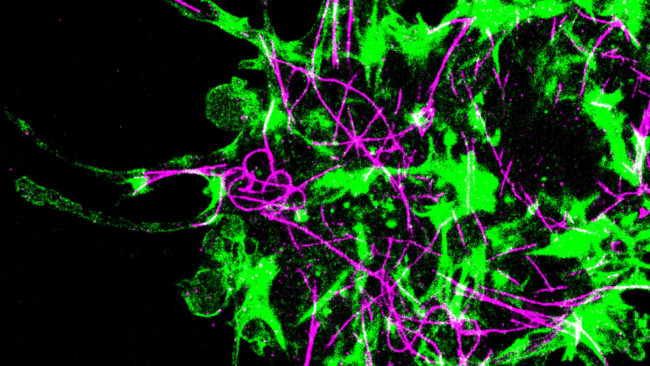
Actin (green) and microtubule (purple) cytoskeletal systems in a single human brain cancer cell treated with an actin inhibitor and interacting with hyaluronic acid, a key component in brain matrix. Cells adhere to the matrix by assembling “microtentacles” that extend tens of micrometers from the cell body and attach via the CD44 receptor.
New research by Berkeley engineers has shed light on how tumor cells adhere to and move through brain tissue. Using long, membranous “microtentacles,” the cells act a bit like Velcro as they digest and work their way through the brain’s lobes.
“Our lab is interested in understanding how cells move,” said Sanjay Kumar, professor of bioengineering and of chemical and biomolecular engineering and a faculty scientist at Lawrence Berkeley National Laboratory. “We used engineered materials to discover a new mechanism through which tumor cells operate. This is particularly important in glioblastoma, a very aggressive type of cancer that can form in the brain.”
The work was published last month in the Proceedings of the National Academy of Sciences.
The average patient survival rate for glioblastoma is about 15 months. Because many affected cells can evade surgery and become resistant to radiation and chemotherapy, learning how and why these cells are so invasive is an important step toward understanding how to stop the invasion itself — and ultimately finding a cure.
As Kumar explained, every tissue in our bodies consists of cells and material that surrounds the cells; this “extracellular matrix” keeps our tissue from falling apart. In our brains, the relevant material is hyaluronic acid (HA). Previous studies used a synthetic polymer material to mimic brain tissue, but Kumar’s team replaced the polymer with the brain’s own HA. Upon doing so, the team observed that a specialized HA-binding cell surface receptor called CD44 allows tumor cells to form very long microtentacles, establishing a foothold that the cells can use to pull themselves forward and shimmy through the tissue — much like a rock climber grabs ahold of a crevice to advance on the rock face.
Study lead author Kayla Wolf, a Ph.D. researcher in Kumar’s lab, made the initial observation that launched the path of the study. “We had identified molecule CD44, which a generation of grad students had modeled for tumor cells to engage with these matrices,” explained Kumar. “But we were missing how that was happening. It was such an important problem, how cells adhere to the brain matrix using integrins, yet almost nothing was known about CD44. Using high-resolution imaging, Kayla dug into this question and discovered that cells have these microtentacles that can extend long distances. We had never seen those kinds of structures before.”
Another of Wolf’s insights was to recognize that such structures had been seen before, just in a different context. In circulating tumor cells, they detached from a primary tumor and entered the bloodstream, advancing metastasis. “She made the connection to that other literature, which was very important to exploring tentacles,” Kumar said. “A few years ago, a different research team observed that glioblastoma tumor cells in tissue don’t exist as isolated cells. Called microtubes, they’re tunnels that provide a mechanism for cells to exchange signals and materials. So we hypothesized that maybe these tentacles are connected or related to microtubes in human brain tumors.”
When Wolf joined the Kumar Lab in 2015, the team had just published a paper on CD44, and she began to look at how it was involved in the invasion process. The human body has a skeletal system to give it structure and muscles to make it move; cells, in turn, have their own skeletal system, called the cytoskeleton, and motor proteins that act as muscles. Because of this, “Cells aren’t just bags of goo, they’re extremely structured with a 3D shape,” Wolf said. Using these mechanisms, cells can apply forces from inside to outside to create a “footlike process” to propel themselves.
Past studies had focused on how different kinds of receptor integrins propelled cell invasion, but CD44 wasn’t appreciated as a receptor that allows adhesion and migration in cells. Kumar’s team began looking at how cells were attaching using the CD44 receptor. By introducing the cell to HA, a softer matrix, they observed the receptor translating information about its surroundings.
“We asked, how are they able to transmit force through this receptor? How do they field different forces?” Wolf said. “We saw that these cells form unique protrusions — microtentacles — that were dependent on this particular receptor. Most protrusions have one type of interaction, but this had two. Glioblastoma cells rarely exit the brain and enter the vasculature — this was a new avenue for microtentacles. And this was the first look at how cells are really interacting with microenvironments.”
The Kumar Lab went on to identify several proteins involved in the process, some of which turned out to be enriched in highly aggressive tumors and could be further explored as disease biomarkers or drug targets.
Other UC Berkeley study co-authors included Poojan Shukla, Kelsey Springer, Stacey Lee, Jason Coombes, Caleb J. Choy, Sam Kenny and Ke Xu.
Funding from the National Institutes of Health helped support this research.
