 Researchers have invented new technology to track RNA, cells, and molecules from a zygote to embryo
Researchers have invented new technology to track RNA, cells, and molecules from a zygote to embryoScientists invent new ways to peer into the earliest stages of life
For many people, the origin of life is still shrouded in mystery. Our knowledge about life’s beginnings seems to end once the sperm penetrates the egg.
But — as you can imagine — a lot happens between fertilization and creation of a living being.
And now thanks to interdisciplinary research by bioengineering professor Amy Herr and a team of engineers and cell biologists, scientists can get a detailed, real time look at the crucial period when genetic molecules like RNA instruct cells on what proteins to form.
“It’s really the next frontier,” Herr said. “We’re looking at life as it is happening, here in a mouse.”
The study — authored by Herr, Elisabet Rosàs-Canyelles, Andrew Modzelewski, Alisha Geldert and molecular and cell biology professor Lin He — was published last week in the journal Science Advances. Conducted with mouse cells, the team’s research used a novel microfluidic technology that could eventually help scientists better understand assisted reproduction technologies (ART) like in vitro fertilization (IVF), as well as lead to improved cancer treatments.
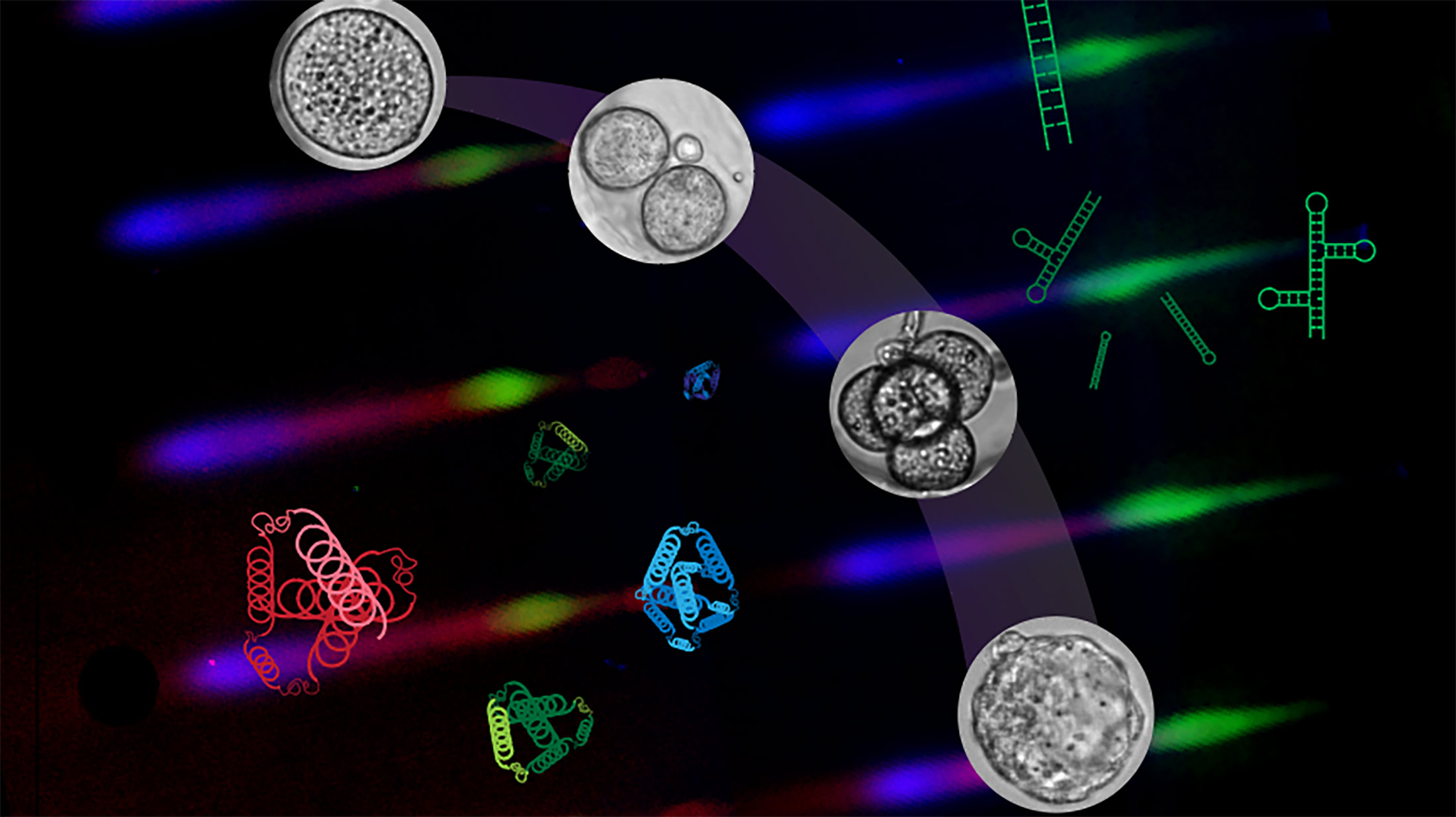
At the earliest stages following fertilization, when a single cell zygote develops into an embryo, researchers don’t have much biological material to work with, Rosàs-Canyelles said.
“There is so little starting cellular material,” she said. “It’s challenging to make accurate measurements.”
In addition, examining the direct relationship between RNA, proteins and cells is a difficult task. In rapidly developing embryos, and in the zygote especially, the time it takes for the RNA’s instructions to manifest in the cell can be an “eternity,” Modzelewski said. “During this dynamic time, the embryo can seem like a black box.”
To observe what’s happening with RNA and corresponding proteins at the same time and in the same cell, the researchers designed microfluidic chips to scrutinize and process single cells using wells built on tiny gel pallets.
So instead of watching an RNA molecule in one cell and then moving onto the related protein in another cell, scientists can simultaneously measure both substances at any given stage of the embryo and in that same cell.
“Then we can figure out what the cells are going to do,” Rosàs-Canyelles said.
Herr said that such levels of precision can reduce the cost and number of research animals needed in developmental biology. The new technology also holds promise for helping women with infertility become pregnant.
Despite advancements in techniques, assisted reproduction technologies like IVF often fail. In both 2018 and 2017, only 24% of full cycle ART procedures resulted in live births, according to data collected by the federal Centers for Disease Control. The numbers are particularly low among women 35 years and older.
The team’s research can help determine why an egg, once a zygote, fails to develop into an embryo by examining the transfer of information from RNA molecules to proteins to a functioning cell.
IVF “can be a gamble,” Modzelewski said. “Our research could help scientists understand why IVF has such a high failure rate. There is still so much we don’t know right now.”
Rosàs-Canyelles, who now works as a scientist with DNA sequencing firm Illumina, said the technology can also help researchers determine how cancer cells respond (or not) to treatments.
More broadly, improving science’s understanding of the earliest stages of life advances our understanding of the world around us, Herr said.
