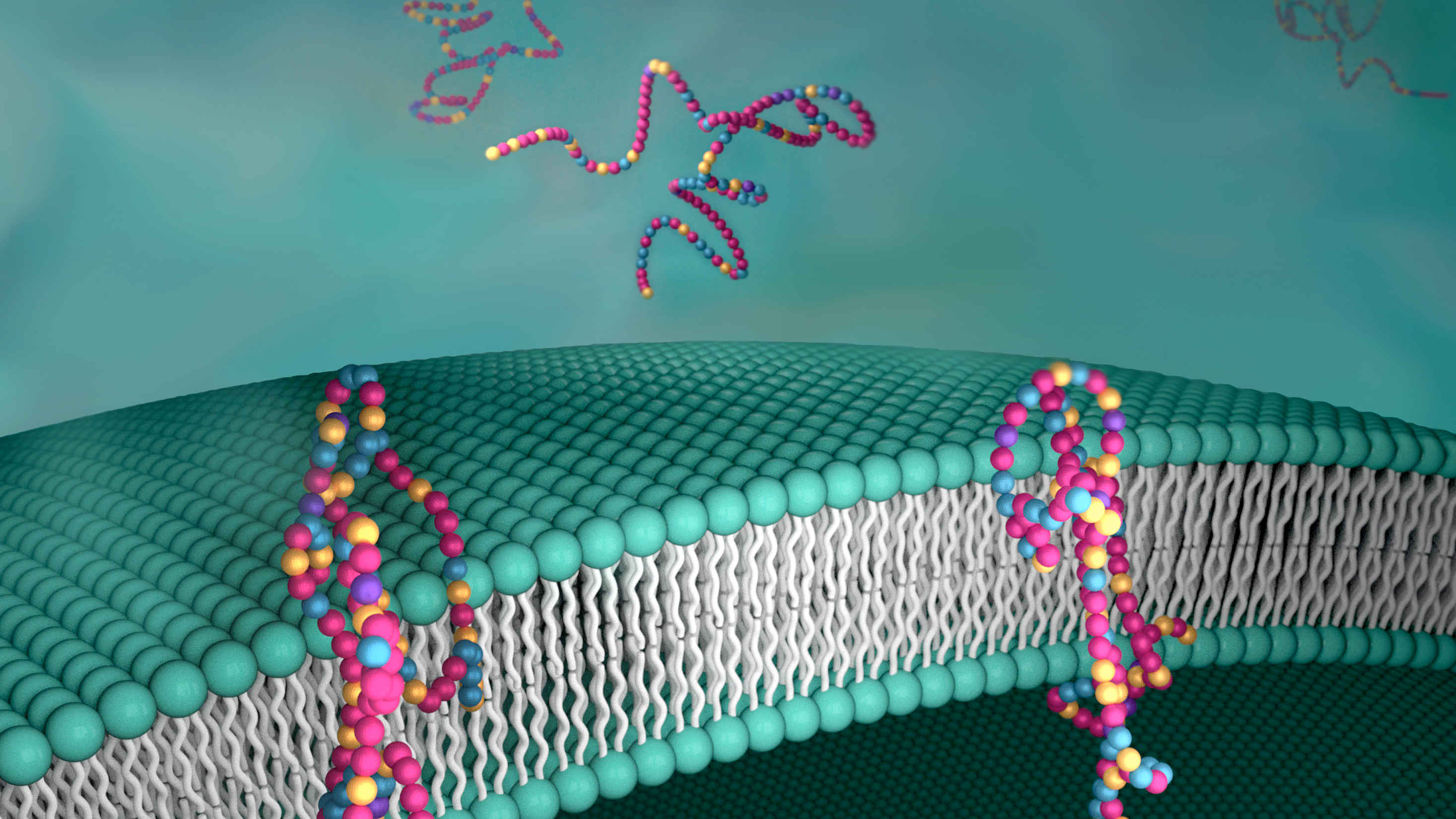
 Scientists have created a synthetic film that mimics transmembrane proteins. (Image by Jill D. Hemman, Oak Ridge National Laboratory)
Scientists have created a synthetic film that mimics transmembrane proteins. (Image by Jill D. Hemman, Oak Ridge National Laboratory)New discovery makes it easier to design synthetic proteins that rival their natural counterparts
Researchers, led by UC Berkeley engineers, have created a synthetic material that is as effective as naturally occurring proteins in transporting molecules through membranes, a major milestone that could transform such fields as medicine, life sciences, alternative energy and environmental science.
Mimicking transmembrane proteins, which act as gatekeepers in living cells, has been a key goal — and a significant bottleneck — in synthetic membrane development for applications such as water desalination, batteries, pharmaceutical and biofuels research. This new achievement, described in a study published today in the journal Nature, could alleviate that jam.
Working with collaborators at UC Santa Cruz, Northwestern University and Oak Ridge National Laboratory, Berkeley engineers designed a polymer that selectively transported protons — charged subatomic particles — across an acrylic film at a rate similar to those of natural proton channels while successfully filtering out other types of cations.
Selective and rapid proton transport is important in the regulation of pH levels and many biological functions. That capability is also useful for clean energy such as fuel cells and energy storage.
“If we think of the polymer as a doorway, it had to be open wide enough for the proton to go through, but not so open that other cations can sneak in,” said study principal investigator Ting Xu, a UC Berkeley professor of materials science and engineering and of chemistry, and a faculty scientist at Berkeley Lab’s Materials Sciences Division. “We were able to find that right balance with our material.”
The paper also provided a solution to a long-standing challenge in designing synthetic proteins that worked like their natural counterparts. Monomer molecules bond together to form polymers, and amino acids are monomers that form proteins. The monomer sequence governs protein function, so for decades scientists believed that they had to copy the monomer sequence exactly to create a synthetic polymer that would function as well as naturally occurring proteins.
Previous attempts to fabricate synthetic protein channels have used sequence-defined polymers — as well as peptides and carbon nanotubes — but none rivaled the performance of their natural counterparts.
Xu and her collaborators found that the monomers in the polymer don’t have to line up nor match exactly to function like a protein. Rather, their design only requires researchers to statistically control the sequence of four types of monomers — Xu calls them random heteropolymers (RHPs) — for the polymer to perform as well as a naturally occurring protein. The monomers can be grouped into segments like Lego pieces to construct functional protein-mimics.
“Compare this to how cars are built,” said Xu. “There are different models, colors and shapes, but they all contain important parts such as an engine, wheels and energy source. For each part, there can be different options, such as gas or electric engines, but at the end of the day, it’s a car, not a train.”
Xu and her team designed a library of polymers that are statistically similar in sequence, providing newfound flexibility in assembly.
“What makes our new technique promising is that it’s scalable, and the knowledge to do this is readily available,” said Xu. “Considering the vast number of monomers available and the recent advances in polymer chemistry, the possibilities of marrying the synthetic and biological fields are almost unlimited. Nature is simple and thrifty. It is our job to crack its code to live in harmony.”
This research builds on previous work by Xu’s team in which RHPs were used as synthetic chaperones to stabilize enzymes in non-natural environments. That work was published in Science in 2018.
Other UC Berkeley co-authors on this paper are Tao Jiang, Aaron Hall, Marco Eres, Yun Zhou, Zhiyuan Ruan, Andrew Couse and Haiyan Huang. Other co-authors include Zahra Hemmatian and Marco Rolandi of UC Santa Cruz; Monica Olvera de la Cruz and Baofu Qiao of Northwestern University; and William Heller of Oak Ridge National Laboratory.
This work was supported by the Army Research Office and the National Science Foundation.
