The fine art of engineering restraint

FIRST IN CLASS: Alumnus Dino Di Carlo (B.S.’02, Ph.D.’06 BioE) was among the first students to enroll in Berkeley’s bioengineering major, established in 1999. Today, he’s on the bioengineering faculty at UCLA, advancing tools for biomedicine. (Photo by Joe Lyman.)
Amid the busy world of Massachusetts General Hospital, Dino Di Carlo (B.S.’02, Ph.D.’06 BioE) experienced a well-known but oft-forgotten truism: technologies need to be simple to have an impact. As a postdoc there, Di Carlo observed that complex diagnostic technologies used in complex biomedical experiments often exacerbated research challenges, resulting in higher data failure rates.
Today, the young assistant professor teaches the art of engineering restraint to his bioengineering students at UCLA and employs it in his research. “Most of the things we’re doing are very, very simple,” Di Carlo explains. “It’s usually one input, a solution of cells. You put them in a channel and image them.”
In September, the National Institutes of Health awarded Di Carlo one of its 2010 New Innovator awards, given to a select number of biomedical investigators for creative, risk-taking projects undertaken early in their careers. (Amy Herr, a Berkeley bioengineering professor, was also honored.)
Accompanied by $1.5 million over five years, the prestigious recognition frees Di Carlo to concentrate on what he and his graduate students do best: tool making. One of their projects, a new design approach for a flow cytometer, promises not only to turn a bulky diagnostic device into a sleek portable unit, but also to drive down the cost of health care and expand access to lifesaving diagnostic equipment.
The complete blood count (CBC) is one of the most common diagnostic tests ordered today. Technicians draw blood from a patient, and the sample is run through a flow cytometer, a high-resolution lab instrument the size of a dorm refrigerator that costs $50,000 or more. Flow cytometers count and examine microscopic particles, most often different cell types, and the data reveal physical and chemical properties that can suggest the presence of everything from allergies and stomach ulcers to cancer.
At the heart of a flow cytometer is a sophisticated fluid pumping system that requires a continuous input of solution called sheath fluid and anywhere from 70 to 1,500 watts to run. As the jumble of blood cells flows through a core stream solution, hydrodynamic forces produced by the pinching sheath fluid “focus” and align the cells in a narrow stream so they can pass through the light from a focused laser beam. By measuring disturbances and optical excitation from the laser light, optical sensors and other imaging instruments identify and count cell types, thousands per second.
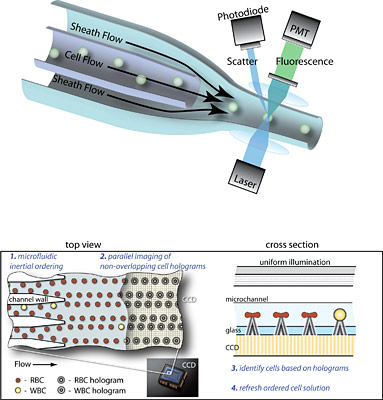
GO WITH THE FLOW: The top figure shows the mechanism of operation for a conventional flow cytometer. In the bottom figure, two different views illustrate how researchers are applying inertial microfluidics to simplify the instrument’s operation to require less fluid and energy. Benefits of this new approach include lower instrument cost and improved access to diagnostic testing.(Graphic by Dino Di Carlo.)
Is there a simpler, better way to design flow cytometers, Di Carlo wondered? Prompted by his experimental observations of particles moving unexpectedly in micro-scale channels, Di Carlo uncovered largely forgotten reports from the 1960s that described how inertial fluid physics led to natural particle migration. Those findings suggested a simpler “cell focusing” technology was possible. Yet no one thought to apply it until Di Carlo put two and two together.
Working with faculty from other universities, the UCLA professor and his graduate researchers developed a technique for injecting a cell sample as one solution—no extra sheath fluid required—that flows through micro-sized channels. The momentum of the fluid and the shape of the channel move and focus the spray of cells passively, without additional energy, into an orderly pattern for the laser beam or other optical excitation. (Di Carlo even observed cells moving across the flow to align themselves, a phenomenon scientists have yet to fully understand.) To further simplify the instrument, the researchers engineered an inexpensive optics system.
One question remained: is a simplified system successful? In initial testing, the team demonstrated that it was possible to analyze 25,000 cells per second, with possible rates up to 49,000 per second. The results compared favorably with current high-speed flow cytometers and met the high-throughput requirements of modern-day diagnostic screening.
Without a sophisticated mechanical pumping system, extra fluid and complex optics, Di Carlo estimates his system will use less energy and cost around $1,000, compared with $50,000 for conventional devices. Reducing high health care costs is certainly one goal, the bioengineer says.
The other is increasing patient access to diagnostic tests and their lifesaving results. Because of its simplicity, Di Carlo’s system could be engineered into a much smaller, portable device, about the size of a laptop computer. His team is currently building a prototype the size of a lunchbox and plans to go as small as an iPhone.
If the technology proves viable, flow cytometers would no longer be confined to a lab. They could easily be available in a doctor’s office or ambulance, carried to a patient’s bedside for point-of-care diagnostics, used in military field hospitals or deployed in developing countries without access to labs. Greater distribution, Di Carlo envisions, means more people will live healthier lives.
The project promises to translate to other fields, too, affecting technologies in tissue engineering, semiconductor electronics and biology. The simple, inexpensive method of precisely controlling microscopic particles “will be revolutionary,” predicts fellow collaborator, Steven Graves, associate professor of chemistry and chemical and nuclear engineering at the University of New Mexico and associate director of the Center for Biomedical Engineering. “Dino has made a fundamental advancement in how we handle cells.”
Graves and other collaborators note how Di Carlo skillfully links academic research with on-the-ground clinical applications. For that, Di Carlo credits the unique education he received in Berkeley’s Department of Bioengineering.
“By taking a broad array of classes in things like signals and systems, neurobiology and continuum mechanics, I learned to speak a lot of different languages and bridge different disciplines,” he explains. Such fluency will serve Di Carlo well as he searches for new ways of engineering simple solutions to complex problems.
